Introduction
Cardiac biomarkers, which include enzymes, hormones and proteins, are biological markers or measurable endogenous indicators that are released into the blood stream when the heart is stressed or damaged. Biomarkers are cardinal indicators in the diagnostic armamentarium of disease trait (risk marker or risk factor), disease state (clinical or pre-clinical) and disease rate (progression or prognosis).
Noting an increase in one or more cardiac biomarkers can assist in the identification of patients with acute coronary syndrome (ACS) and acute myocardial infarction (AMI), allowing rapid diagnosis and treatment.
Cardiac biomarkers tests
Cardiac biomarker testing aids in detecting the presence of ACS and AMI and the evaluation of its severity, so that appropriate therapy can be administered to the patient. It is important to differentiate a heart attack from angina, heart failure or any other condition, since treatment and monitoring requirements are different for each condition. As time is muscle, prompt medical or surgical intervention is vital to minimise heart damage and future complications. Essentially, cardiac biomarker tests ought to be accessible quickly to doctors – 24 hours a day, seven days a week.
The tests may be performed at point of care, in the emergency room, or at the patient's bedside. Serial testing of one or more cardiac biomarkers is advised, to ensure that their upsurge in blood levels is not missed and to estimate the severity of a heart attack.
Normal values for cardiac biomarkers are as follows:
Creatinine kinase (CK): 20-192 units/l
Creatinine kinase myocardial band (CK-MB): 0-6 ng/ml
High-sensitivity cardiac troponin T (hs-cTnT): <14 ng/l, or I (hs-cTnl): <17.4 ng/l
High-sensitivity C-reactive protein (hsCRP): 0-3 mg/l
The troponin T or I will start to rise three to four hours after injury and can stay elevated for up to two weeks. Within the normal healthy population, 99% will have an hs-cTnT (@ Mount Elizabeth Hospital < 14 ng/l) or hs-cTnI (@ National University Hospital < 17.4 ng/l). Cardiac troponins peak between 24 and 28 hours after initiation of infarction. Values above 30 ng/l are more likely to be consistent with a myocardial infarction (MI). The higher the level the more likely the patient has had an MI, although stable levels marginally above 30 ng/l do occur with alternative pathology.
At the European Society of Cardiology (ESC) Congress on 1 September 2019, it was shown that a rapid assessment algorithm based on hs-cTn and sampling at 0 and 1 hour is recommended to rule-in and rule-out AMI. Serial CK-MB and troponin T or I are important. Myoglobin is detectable in the serum after AMI. It is one of the earliest cardiac biomarkers to increase in concentration in the blood following AMI. However, there are many other causes of raised myoglobin in serum and urine. As such, it is not currently in the guidelines for diagnosis.
D-dimer
D-dimer testing is of clinical use when there is a suspicion of deep venous thrombosis, pulmonary embolism or disseminated intravascular coagulation. It is under investigation in the diagnosis of aortic dissection. The reference range of D-dimer is 0.00-0.49 ug/ml.
B-type natriuretic peptide
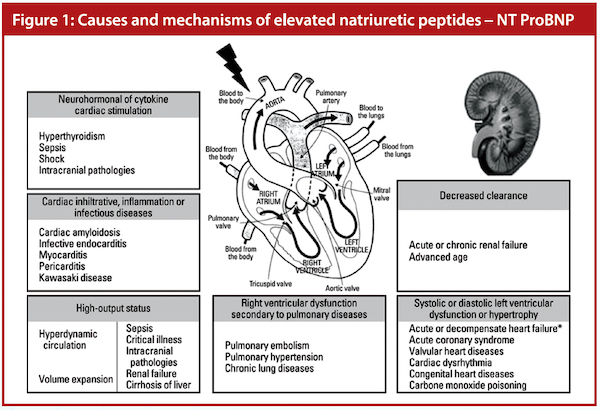
B-type natriuretic peptide (BNP) belongs to a family of naturally occurring hormones known as natriuretic peptides and are produced by the heart. The N-terminal-pro BNP (NT-proBNP) is a non-active pro-hormone which is released from the same molecule that produces BNP. Being synthesised in the cardiac ventricles, both BNP and NT-proBNP are liberated in response to changes in the pressure inside the heart. These changes can be linked to heart failure or other cardiac events. The levels of BNP and NT-proBNP go up when heart failure develops or gets worse, and their levels drop when heart failure stabilises. Being a relatively specific biomarker for cardiac dysfunction, BNP and especially NT-proBNP are increasingly being used in the evaluation and management of heart failure. ESC guidelines recommend a Class 1a use of BNP/NT-proBNP concentrations as a key element in the early diagnosis of heart failure.
The US Food and Drug Administration approved a cut-off value of NT-proBNP for the diagnosis of congestive heart failure (CHF) as 100 pg/ml in 2019. (A normal value in Parkway Laboratory is 0 to 100 pg/ml.) In NT-proBNP, the optimal cut-off values for confirmatory (rule-in) decision limits for CHF are 450 pg/ml for ages less than 50 years, 900 pg/ml between 50 to 75 years, and 1,800 pg/ml above 75 years of age. The exclusionary (rule-out) decision limit of NT-proBNP for cardiogenic acute dyspnoea in all ages is less than 300 pg/ml. Natriuretic peptides are useful markers in differentiating acute dyspnoeic patients and are potent prognostic markers for CHF.
 Source: Scientific figure on ResearchGate. Available at: https://bit.ly/3tGHkHj.
Source: Scientific figure on ResearchGate. Available at: https://bit.ly/3tGHkHj.
New cardio biomarkers in the pipeline
Cardiovascular disease is one of the leading causes of mortality in the world. Thus, there is an emerging need for rapid diagnosis and intervention for management of ACS. This, in turn, is continually propelling investigations into potential molecular cardiac biomarkers. Several candidate molecules are in the discovery and development pipeline, including growth differentiation factor15, soluble suppression of tumorigenicity 2, heart-type fatty acid binding protein among others.
Although most cardiovascular biomarkers are used by clinicians without taking gender into account, sex-specific differences in biomarkers evidently exist. Women and men differ in their cardiac physiology and manifestations of cardiovascular disease.
Baseline concentrations of most common biomarkers (including cardiac troponin and natriuretic peptides) differ between men and women, but these sex-specific differences do not usually translate into a need for differential sex-based cut-off points. Furthermore, most biomarkers have similar diagnostic and prognostic implications regardless of the sex, with the potential exception of cardiac troponins measured through high-sensitivity assay. Troponin levels are lower in women than in men and, with the use of high-sensitivity assays, sex-specific cut-off points may improve the diagnosis of ACS.
In addition, proneurotensin is a novel biomarker that has been found to be predictive of incident cardiovascular disease in women but not in men, and was also predictive of incident breast cancer. If this is confirmed, proneurotensin might be a unique biomarker of disease risk in women.
With any biomarker, an understanding of sex-specific differences might improve its use and might also lead to an enhanced understanding of the physiological differences between the hearts of men and women.
About 70% of cardiovascular disease (CVD) cases and deaths are attributed to modifiable risk factors, which could be predicted through history-taking and cardiac-biomarker testing, allowing the individuals concerned to alter lifestyles and address the risk factors. These biomarkers provide important prognostic information in addition to clinical parameters. Additional diagnostic tests such as serial ECGs, treadmill stress tests and chest X-rays; imaging modalities including echocardiography and stress echoes; and perfusion studies such as technetium-99m sestamibi or myocardial perfusion imaging, rubidium positron emission tomography scans and computer tomography coronary angiograms, allow proper diagnosis and effective management.
Damage to the heart muscle cells from ACS is the classic cause of elevated troponin. Although cardiac troponins have been accepted as the gold standard in the diagnosis and risk stratification of ACS, misinterpretation of detectable troponin levels in the emergency department or other in-hospital settings may lead to confusion in terms of diagnosis and choice of suitable therapy options. Physicians should be aware of the non-ischemic causes of troponin positivity as well as their pathophysiology and clinical impact to prevent unnecessary invasive and non-invasive treatments and coronary-care-unit admissions.
There are many other (albeit minor) causes for elevated troponin levels, among them acute and chronic heart failure, myocarditis and cardiac contusion from trauma. There are also many non-cardiac causes of increased troponin levels, such as renal failure, pneumonia and pulmonary embolism.
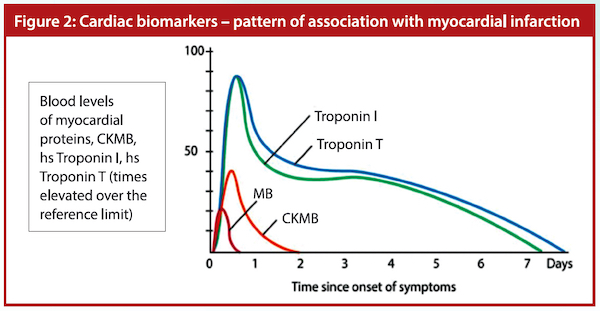 Adapted from ECG & Echo Learning – Diagnostic Criteria for Acute Myocardial Infarction: Cardiac troponins, ECG & Symptoms. Available at: https://bit.ly/3y7UP6l.
Adapted from ECG & Echo Learning – Diagnostic Criteria for Acute Myocardial Infarction: Cardiac troponins, ECG & Symptoms. Available at: https://bit.ly/3y7UP6l.
COVID-19 and the heart
The morbidity and mortality in COVID-19 are primarily related to respiratory and/or circulatory failure. The respiratory distress in severe COVID-19 cases may be the result of the combination of lung injury (pneumonia/acute respiratory distress syndrome) and heart failure due to ACS or myocardial injury.
Dr Allan Jaffe of Mayo Clinic stated that high-sensitivity troponins T (hsTnT) or I (hsTnI) may be elevated in COVID-19 patients whose hearts are affected by SARS-CoV-2. In some cases, 25% to 30% elevation of hsTnT is possible. In the majority of cases where non-ST-segment elevation myocardial infarction is the diagnosis, the management should be conservative. If plaque rupture is suspected or there is ST-segment elevation myocardial infarction, then coronary angiography and percutaneous coronary intervention may be done with utmost precautions with personal protective equipment. Both short-term and long-term prognosis may be worse in these classes of patients.
Dr Dara K Lee Lewis, co-director of the women's cardiology programme at Lown Cardiology Group, stated that pre-existing heart conditions and poor metabolic health increase risk of severe COVID-19, compared to the general population.
Diabetes increases risk of severe COVID-19 by suppressing the immune system, while other illnesses like asthma increase risk by weakening the lungs.
People with CVD were more than twice as likely to contract severe forms of COVID-19. There are two explanations as to how CVD increases the risk of severe COVID-19. The first is that pre-existing heart conditions, such as damaged heart muscle or blocked heart arteries, weaken the body's ability to survive the stress of the illness. A person with a vulnerable heart is more likely to succumb to the effects of fever, low oxygen levels, unstable blood pressures and blood clotting disorders (all possible consequences of COVID-19) than someone previously healthy.
A second explanation relates to poor underlying metabolic health, which is more common in those with heart disease. Poor metabolic health refers to diseases such as type 2 diabetes or prediabetes and obesity, which themselves cause inflammation and risk of blood clots, compounding the effects of COVID-19 and increasing the likelihood of devastating complications of COVID-19.
Increased cardiovascular risks with COVID-19
COVID-19 can damage the heart in several ways. The virus may directly invade or inflame the heart muscle, and it may indirectly harm the heart by disrupting the balance between oxygen supply and demand. Heart injury, which may be measured by elevated hsTnT in the bloodstream, has been detected in about one-quarter of patients hospitalised with severe COVID-19 illness. Of these patients, about one-third have pre-existing CVD.
The majority of people with COVID-19 will have mild symptoms and recover fully. However, about 20% will develop pneumonia and about 5% will develop severe disease. In the severe form of COVID-19, the body's immune system overreacts to the infection, releasing inflammatory molecules called cytokines into the bloodstream. This "cytokine storm" can damage multiple organs, including the heart.
Myocarditis typically occurs only in advanced COVID-19 disease. Myocarditis can result from direct heart invasion by the virus itself, or more commonly by inflammation caused by the cytokine storm. When this occurs, the heart may become enlarged and weakened, leading to low blood pressure and fluid in the lungs. While this severe form of myocarditis is rare, recent studies have suggested that a milder form of heart muscle inflammation may be much more common than previously recognised. A recent study showed that asymptomatic heart inflammation was seen on MRI in up to three-quarters of patients who had recovered from severe COVID-19. Increased oxygen demand and decreased oxygen supply lead to heart damage.
Fever and infection cause the heart rate to speed up, increasing the work of the heart in COVID-19 patients who develop pneumonia. Blood pressure may drop or spike, causing further stress on the heart, and the resulting increase in oxygen demand can lead to heart damage, especially if the heart arteries or muscle were unhealthy to begin with.
Heart damage is most often caused by heart attacks, which result from the formation of a blood clot in a vulnerable heart artery, blocking delivery of oxygen to the heart muscle. COVID-19-related inflammation raises the risk of this type of heart attack by activating the body's clotting system and disrupting the blood vessel lining. When inflamed, this lining loses its ability to resist clot formation. These blood clots in the arteries of the heart cut off its supply of oxygen. The increased clotting tendency can also lead to blood clots in the lungs, which can cause a drop in blood oxygen levels. Severe pneumonia drops blood oxygen further. When the oxygen demand exceeds supply, the heart muscle is damaged.
People with CVD who adopt adequate physical activity and a healthy diet can strengthen their defences and reduce any long-term risk of CVD from COVID-19.
Getting protected from COVID-19
Vaccination is important to protect all and create herd immunity. Vaccination generates both the desired antibodies and the immune cells, the T-cells. There are many players in this field: Pfizer-BioNTech, Moderna, Oxford-AstraZeneca, Russia's Sputnik V, China's Sinovac and India's Covaxin. The first two have 90% to 95% protection while the other three claim more than 85% protection. All of them need two doses and special storage facilities. Moderna and Pfizer-BioNTech are novel types of vaccines made from messenger RNA (mRNA) and are effective against the original and probably the more contagious mutated strains which appeared in South Africa, Brazil, the UK and India. Mutations in the virus are due to random changes in its genetic sequence. Messenger technology can directly start to engineer a vaccine which completely mimics this new mutation.
The mRNA vaccine involves injecting only fragments of the genetic material. When these viral genetic fragments enter the human cell, they commandeer the cell to produce the signature protein of the coronavirus. This trains the body to recognise the key part of the virus – the spike protein, without exposure to the whole virus. This contrasts with previous methods where the protein itself is used, which usually takes years to manufacture the vaccine, but which recently only took two to six weeks.
In January 2021, two new vaccines from the US, Johnson & Johnson's single dose vaccine and Novavax have come into the market. Ideally, a universal vaccine effective against all variants and mutations of COVID-19 should be available in the near future.