I recently received the Pfizer-BioNTech COVID-19 vaccine. After the painless injection, I waited outside the room for 30 minutes in case of any severe allergic reactions. As there were none, I registered to return in three weeks' time to receive my second dose.
Eight hours after the injection, I felt a slight ache in my arm but when I examined the injection site, there was only the red mark of the needle entry and no bruising or swelling. I felt warm but when I checked my temperature, it was 36.8°C. I was otherwise totally asymptomatic. Phew!
Three weeks later, I received the second dose. The side effects were identical to the first dose, that is, minimal.
My colleagues who received the vaccine also complained of arm pain. Some had fever, tiredness, headaches, dizziness and chills which lasted for a day or two. Fortunately, nobody I know had any severe allergic reactions.
Early history of the COVID-19 vaccines
Like many kiasu doctors, I did some research on the different vaccines before accepting this vaccine. It is true that the COVID-19 vaccines were achieved in record time, under one year. Some say this was too rushed, and point to some severe reactions.1 There are two aspects to vaccine development: laboratory research and animal tests, and clinical trials.2
The first coronavirus was identified in 1965, but vaccine development did not start until the SARS epidemic in 2003. Unfortunately, research stalled because the outbreak evaporated within a year. The same thing happened again with the MERS outbreak in 2012.3 Singapore and other countries joined in the vaccine research, but once again the outbreak subsided and research was suspended, never progressing to clinical trials.
Katalin Kariko and Drew Weissman
Groundbreaking research that later emerged to become the foundation of the current messenger RNA (mRNA) vaccine was conducted initially without any connection to the vaccine. Notable is the work of Katalin Kariko and Drew Weissman, who jointly developed the ability to translate mRNA into predetermined proteins and to insert the mRNA into cells. The history of Kariko's work is tantalising.4 In the 1980s, she joined the University of Pennsylvania and proposed that mRNA could be used not only to fight diseases but also for vaccine development. Sadly, the university consistently rejected her unorthodox ideas and research grant applications, and even demoted her in 1995 from her academic position; at the same time, she was diagnosed with cancer. Luckily, Kariko's colleague Weissman believed in her ideas and collaborated with her. Kariko did not resign from her position, but persevered with their research which was published in 2005. Eventually, her prescient idea of an mRNA vaccine was recognised by others, and later by two pharmaceutical companies – BioNTech and Moderna.
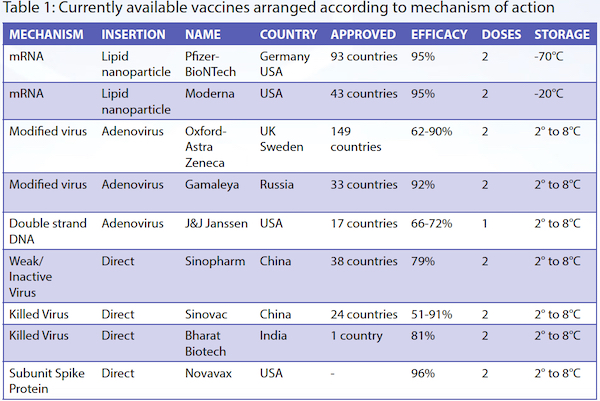
Mechanisms of action of different vaccines5,6
mRNA vaccines
A special coded message in the mRNA is presented to the ribosome, which will then translate the message to manufacture the desired protein antigen – in this case, the spike protein found on the COVID-19 virus. Kariko's contributions were crucial. Firstly, she produced a lipid nanoparticle coat that enables the mRNA to penetrate the cell membrane of macrophages and dendritic cells found close to the injection site. Secondly, she discovered exactly how to rewrite the mRNA to give a precise instruction for the cell to produce the immunogenic protein.
mRNA vaccines are advantageous as no virus is used, so one will not come down with COVID-19. The vaccine mRNA does not enter the cell nucleus and therefore cannot affect the genetic material inside the nucleus.
Pfizer-BioNTech and Moderna adopted this vaccination technique, and each completed a Phase 3 double-blind placebo-controlled trial. By December 2020, they confirmed its safety with minimal side effects, and achieved 95% efficacy. The US Food and Drug Administration quickly gave emergency approval. The Pfizer-BioNTech vaccine needs to be stored below -70°C, and the second dose is given three weeks later,7 while the Moderna vaccine has to be stored at -20°C, with the second dose given four weeks later.8
Weakened virus inserted into another viral vector
The use of the adenovirus as a vector to help insert the genetic material of a virus into a cell has previously been employed for measles, Ebola, Zika and HIV vaccines. The adenovirus DNA is first removed, leaving an emptied protein coat or capsid. This is then filled with the intended SARS-CoV-2 virus which has been weakened or inactivated. The adenovirus capsid helps insert the inactivated coronavirus into the cells, thus mimicking a viral infection.9
Oxford-AstraZeneca chose to use a chimpanzee adenovirus as its vector. In an editorial published in the November 2020 issue of Cell, adverse side effects include "fever, pneumonia, diarrhea, transient neutropenia and lymphopenia, fatigue, labored breathing, headaches, liver damage, and fasting hyperglycaemia. Rare adverse reactions include blood clots affecting the abdomen or brain,10 neuropathies such as Bell's palsy, Guillain-Barre syndrome, gait disturbance, and transverse myelitis." So far, the Oxford-AstraZeneca trial results show 70% efficacy,11 and the Russian Sputnik V vaccine, which uses the same technique, is said to be 91.4% effective.12 Both vaccines need to be stored between 2°C to 8°C.
Double strand DNA inserted into adenovirus
Johnson and Johnson's vaccine differs from other vaccines in that it uses DNA and not RNA. A doubled strand of DNA is inserted via an adenovirus vector. The DNA enters the cell's nucleus and carries instructions to manufacture a special non-viral mRNA, which in turn translates the instructions to manufacture the spike protein of the SARS-CoV-2 virus. The other difference of this vaccine is that it only requires a single dose. The vaccine is undergoing Phase 3 clinical trials, and to date the efficacy is between 66% to 72%. It is stored between 2°C to 8°C.
Weakened viruses injected directly
The Salk polio vaccine, most influenza vaccines and the hepatitis A vaccine use the technique of weakening or inactivating the virus which is then injected directly into the subject. This technique has been employed in two of the China-produced vaccines, Sinopharm's BBIBP-CorV and Sinovac's CoronaVac,13 and India's Bharat Biotech's Covaxin.14 These "killed" virus vaccines can be stored between 2°C to 8°C. Some commentators are uncertain about the efficacy and side effects of these vaccines.
Subunit vaccines
The Novavax vaccine takes a purified piece of the spike protein of the SARS-CoV-2 virus, and adds an adjuvant which enhances the immunogenicity of the spike protein. Both the spike protein and adjuvant are injected into the subject. So far, the immune response in terms of antibody production as well as increased T-cells have been promising. However, Phase 3 trial data has not been released to date. Novavax can be stored between 2°C to 8°C.15
The information above is summarised in Table 1.
Vaccine hesitancy
Many people have a fear of needles stemming from medical injections over the years. Compounded by over-dramatised information, such as anaphylactic shock and deaths following vaccinations, there is a reluctance for some people to voluntarily accept the vaccines. Most of these severe side effects and deaths have been shown to be unrelated to the vaccine.16
Severe allergic reactions to the vaccine do occur, but they are very rare and are found in those who have a past history of severe allergic responses.17,18 Those who have a history of such reactions are advised not to take the vaccine. These severe responses usually occur within 15 minutes of the vaccination, and hence vaccinated subjects are observed for 30 minutes after the injection.
Even in Singapore, a survey conducted on 26 April 2021 showed that 67% are willing to accept the free vaccination offered by the Government.19 Perhaps Singapore may be a victim of its own success.20 It has famously flattened the infection curve and reduced the local spread to less than five per day, sometimes with zero cases. The total death rate in the past year reached 32 (as of print), which contrasts with the thousands of daily deaths in most other countries. This has led some Singaporeans to believe that since there are virtually no locally transmitted cases of COVID-19, there is no benefit to getting vaccinated. The Mayo Clinic outlines the benefits.21
While Singapore may be doing well in controlling the spread of coronavirus, the world is still teeming with millions of cases. Travellers to Singapore will inevitably bring some of the virus to our country. There is another potential problem; namely, new mutations.
New mutations
New mutations of the virus have been discovered in the UK, Brazil, South Africa22 and India.23 They have been found to be highly infective, probably due to better binding of the virus to the Angiotensin Converting Enzyme 2 receptor, which increases a person's susceptibility to the virus. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases has stated that the British new mutant variant is more deadly than the original virus.24
At the moment, the Pfizer-BioNTech and Moderna vaccines seem capable of stimulating a vaccinated person's antibodies to neutralise the invading mutant variants.25,26
Prof Ooi Eng Eong of Duke-NUS Medical School told the Straits Times that "Singaporeans need not worry about the mutations being reported or the possibility of having to alter the vaccine in the near term."26
But we cannot sit on our derrieres. Although virologists believe it is unlikely, it is not impossible in the future for a virus to mutate into a new strain which is resistant to the current vaccines.
Who should receive the vaccine?
It is recommended that everybody should seriously consider receiving the vaccine. Those who have had severe allergic reactions in the past, pregnant mothers, children under the age of 12* years and severely immunocompromised people are not recommended to receive the vaccine. Those who have already contracted COVID-19 are recommended to still receive the vaccine.
The Singapore Government promises that all citizens will have access to the full two doses of vaccines, and even long-term residents will also be eligible.27 The Islamic Religious Council of Singapore holds the position that a COVID-19 vaccine is permissible for Muslim use. The recommended timing of the second vaccine dose varies slightly according to the vaccine manufacturer.
Final thoughts
At the time of writing, we do not know the effectiveness of the vaccines because clinical trials were only started in the middle of 2020. Our past experiences with other vaccines remind us that no vaccine is 100% protective. We also do not know how effective the vaccines are against the new mutant variants.
The vaccine is probably our best chance of controlling the pandemic. By taking the vaccine, you become immune to the SARS-CoV-2 virus, which means that not only will you be protected, but you will not transmit it to others. If more than 80% if the population is protected, then this is effective herd immunity.
In the meantime, please continue to wear masks, observe social distancing and keep clean. The benefits of the vaccine will trump the side effects. My advice is: "Go for it!"
 Source: https://nyti.ms/3hu5OBl
Source: https://nyti.ms/3hu5OBl
Information in this article is accurate as of 21 May 2021.
*Singapore has approved the Pfizer-BioNTech vaccines to be given 12 years and above.