Clinical research, including clinical trials, is the foundation for evidence-based medicine and is critical for the discovery of new treatments and prevention methods. In the past decade, the protection of human rights, and the safety and welfare of research participants, is seen as being increasingly important in clinical research studies. Clinicians and researchers now spend a considerable amount of time on "administrative matters" to comply with requirements set by regulatory agencies and funding bodies. In the era of big data with personal data protection laws and transparency obligations, regulatory requirements are complex. The sharing of data and use of participants' data for future research require participants' consent or anonymisation/ de-identification to protect their privacy. Following the direction taken by countries with well-established biomedical research sectors, such as the US, the UK and Australia, the Ministry of Health recently implemented the Human Biomedical Research Act (HBRA)1 on conducting human biomedical research and handling of human tissue.2,3
In this article, we focus on the challenges faced by researchers in complying with the recent regulations in biomedical research in Singapore. The HBRA requirements on appropriate consent for use of identifiable Health Information (HI) or human biological materials (HBM) and de-identification have caused significant confusion and delays in the recruitment and progress of research studies.
Informed consent vs appropriate consent
Traditionally, informed consent was designed to deal with a single study, with a specific purpose and a pre¬defined time span. The goal of the informed consent process is to clearly convey all information pertaining to the research study to the participants to help them understand and voluntarily provide their consent for participation. However, with the rise of big data and data sharing, data collected could be kept for long periods of time, used in multiple studies with different research purposes, linked to heterogeneous sources and shared widely with other investigators. To allow for future use, in the "appropriate consent", separate consents for re-identification in case of incidental findings and future use of data and tissue have been introduced.
With the introduction of appropriate consent, explaining additional consent elements has made the consent process longer (an increase from approximately 15 minutes to approximately 30 minutes) and more difficult to understand – both of which adversely affect participants' overall experience in the research study and increase their unwillingness to participate in future studies or follow-up visits. The many components in the appropriate consent form render participants, especially the elderly, confused, apprehensive and doubtful of the integrity of the research. For example, explaining the terms "giving up intellectual property rights" and "human-animal combination" could result in them refraining from participating in studies even with minimal risk. Participants often feel overwhelmed by the information overload and the process is stressful for most of the Asian participants who do not have adequate health literacy as compared to Westerners.
Obtaining re-consent
Obtaining re-consent from participants for blood/urine collection (interventional) in ongoing studies that had already commenced recruitment using the existing "Core Consent Elements" may likely cause anxiety in some participants who may feel that their privacy has been violated, in addition to the extra time and resources required to obtain re-consent. Since the activation of the HBR framework in November 2017, several versions of the consent forms have been released by the Institutional Review Board, and this has led to significant amount of time and effort being expended on amending and tracking the various versions.
Collaboration and data sharing
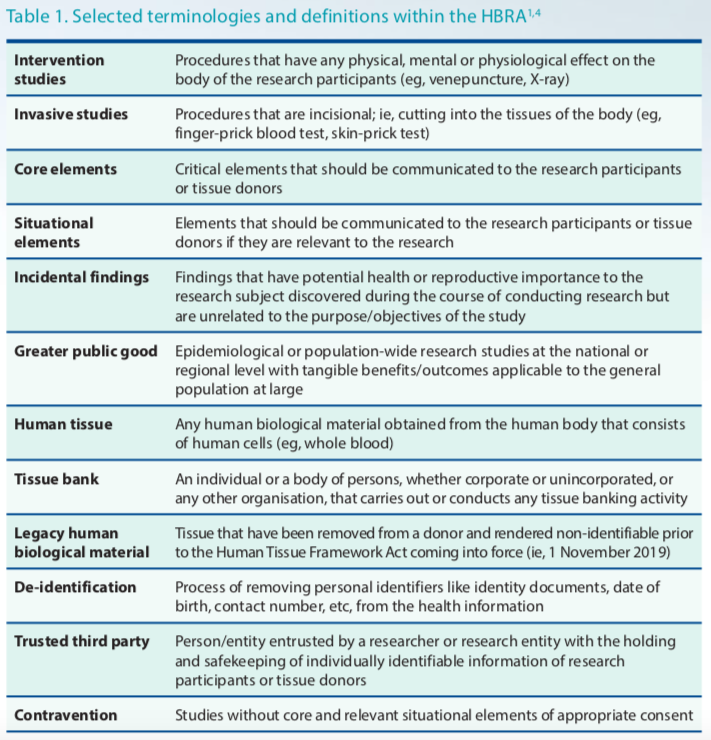
The requirement to have a trusted third party and to de-identify HI/HBM for analysis also needs additional manpower and funding, resulting in several ongoing studies suspending recruitment to complete de-identification. Under the new Human Tissue Framework, sharing of samples with collaborators requires researchers to perform tissue banking activities, which require another set of requirements to comply with. This will also affect collaborative research. Understanding and interpreting the definitions and terminologies (Table 1) was daunting for researchers (eg, definition of "tissue bank" in the context of planning future use of tissue at the outset versus storing leftover tissue for future use).
With large collaborative grants, initiatives such as data sharing and data federation have been gaining momentum lately. Large-scale data sharing across disciplines and countries expand the value of research by enhancing scientific discoveries and facilitating validation of results that benefit individual as well as population health. However, data sharing comes with substantial challenges, such as developing a data sharing plan at the outset of the project, deciding on the type of agreement needed to get the data (eg, research collaboration, project agreement, material transfer agreement and data sharing agreement), and getting consensus from concerned institutions' administrative and legal consultants on data sharing. As execution takes a long time, data harmonisation and analysis are also delayed. This will in turn impact the research projects' milestones and hinder researchers from completing the project within the stipulated grant period.
In conclusion
Biomedical and clinical research in Singapore is relatively new, and the research landscape and regulatory requirements are evolving rapidly. Researchers in Singapore may find it difficult to adopt to the swift legislative changes, and be overwhelmed by the required administrative paperwork and the need for additional resources for implementation of research. These changes lead to greater stress among researchers and divert their focus from effectively performing core research activities such as applying for grants, conducting the actual research, publishing papers, mentoring students and translating clinical research to practice.
HBRA has the potential to protect the rights, safety and welfare of subjects who donate or participate in research, and demonstrate Singapore's commitment towards ethical and responsible biomedical research and handling of human tissue. Researchers who are part of big research teams, with dedicated administrative teams and funding support, will adapt to the changes in due course, but it would likely deter early-career researchers or smaller institutions that do not have adequate manpower from participating in research. As the extensive and complicated consent process often exceeds the capacity of participants to understand their entitlement, raising public awareness about informed consent would ensure that participants are making informed decisions and that their rights are protected. Ultimately, the public and society need to recognise the importance of clinical research in promoting health and improving treatment.